Research Paper 1: Diabetes Mellitus
Lieberman, Leslie Sue Lieberman. “Diabetes.” Credo Reference Home. Cambridge University Press, n.d. Web. 13 Apr. 2013. <http://www.credoreference.com/topic/diabetes
Diabetes mellitus (DM) is an endocrine disorder characterized by the lack or insufficient production of insulin by the pancreas. Insulin, a hormone produced in the pancreas by the islets of Langerhans regulates the amount of glucose in the blood. The lack of insulin causes a form of diabetes. DM has been recognized as a disease for at least two millennia, but only since the mid-1970s has there been a consensus on its classification and diagnosis. Symptoms include excessive urination, urine containing sugar, hunger, thirst, fatigue, and weight loss, are common to all types of DM.
The primary diagnostic criterion for DM is elevation of blood glucose levels during fasting or at 2 hours following a meal. Normal plasma glucose values for adults in the fasting state are 80–120 milligrams per deciliter (mg/dL). Definition of unequivocal DM requires a 2-hour postingestion plasma glucose level equal to or greater than 200 mg/dL for the appearance of classical symptoms of diabetes. Despite the use of a plethora of different terms in the past, diabetes is now generally classified as type I DM (insulin-dependent diabetes) and type II DM (non-insulin-dependent diabetes). Other variants of DM include maturity-onset diabetes of youth, tropical diabetes, which shows characteristics of both insulin dependence and nondependence, and gestational diabetes, which occurs during the latter part of pregnancy. Approximately 90–95 percent of all diabetics may be classified as type II, and about 5 percent as type I. Some 2 percent of diabetics have DM as a secondary result of other disease or injury.
Insulin-Dependent Diabetes Mellitus (Type I) Insulin-dependent DM is characterized by clinically acute onset, usually at an early age, reduction in the production and excretion of insulin, weight loss, thirst, frequent urination, and high levels of blood sugar. Some nutritional factors have been suspected of being involved in the etiology of type I diabetes, although there is no consistent picture. There is strong evidence for a genetic susceptibility, but of those individuals who carry the suspected antigens, only 30–50 percent develop DM; thus, environmental factors also appear to have a role. A number of studies indicate an increased prevalence of type I diabetes among populations that previously showed low rates. Excess caloric intake does not seem to be the important factor it is in type II diabetes. Typical type I diabetes is uncommon, affecting less than 0. 5 percent of the world’s population. Other possible causative factors include infectious viruses such as mumps, rubella, and meningitis. In general, these increases are associated with a “Westernization” of lifestyle since World War II.

Type 1 Diabetes
Abundant worldwide evidence associates obesity with type II diabetes, and experts have concluded that it is the most powerful risk factor for non-insulin-dependent DM. However, it is difficult to disentangle the effects of decreased fiber consumption from increased sugar and carbohydrate intake, increased total calories, total fat, decreased caloric expenditure, and stresses associated with rapid dietary change and modernization. Conversely, during World War I and II in Europe and during World War II in Japan, when caloric intake was markedly decreased, obesity and diabetes both declined. It has been suggested that dietary fiber decreases the risk for diabetes, and different forms of fiber are being investigated in this connection. Genetic mechanisms interacting in complex ways with environmental factors are involved in the risk for type II diabetes. Diabetes rates have increased in a number of countries, such as Japan, Taiwan, Haiti, New Guinea, and parts of Africa, where caloric consumption per capita has also increased. Many causative factors have been implicated in type II diabetes and many observations have been made on sex differences in the frequency of type II diabetes. Most researchers agree that there is no convincing evidence that a single dietary component increases the risk of diabetes. More recent studies documented high frequencies of both obesity and diabetes among a number of Amerindian tribes in Oklahoma and in Latin American populations.

Type 2 Diabetes
Tropical Diabetes, A type of diabetes found primarily in many tropical areas of the world has characteristics of both type I and type II. However, many areas show high rates of diabetes in populations that do not consume cassava, and some populations have high cassava consumption and low rates of diabetes. Risk factors for tropical diabetes involve unique dietary items. Information is relatively sparse on the genetics of diabetes in tropical countries. Recent studies have shown great population variability in increased susceptibility to diabetes. Genetic studies of Indian populations suggest a stronger familial factor among them compared to diabetics in other populations. For example, some types of cassava (manioc) may be toxic and produce pancreatic damage. In Kenya, a local alcohol called changaa is implicated in causing the disease. Finally, in most tropical areas carbohydrates constitute 70–80 percent of total calories, and such a diet is implicated in classic malnutrition diabetes because of low nutrient density and high fiber content.
In general, cities in the United States report a higher prevalence of gestational diabetes than do European cities. This form of the disease is difficult to distinguish from type II diabetes because a woman could have diabetes before pregnancy but not have it diagnosed until pregnancy. A type of diabetes present only during pregnancy was noted in 1882, which was Gestational diabetes. Babies born to diabetic mothers usually are large but may have immature organ systems, in which case they may not survive. However, it was not until the 1940s that the term “gestational diabetes” appeared in medical literature. The highest reported rate of gestational diabetes occurs among the Pima Indians of Arizona, who also have the highest prevalence of type II diabetes of any known population.
The publish paper from the University of Cambridge gives a historical side to diabetes. It does not go into any form of detail information on the chemistry of the disease but the implications. My reasoning for choosing this paper is due to the hormone Insulin. Insulin is a chemical messenger which contains several Amino Acids, 51 to be exact, which codes for its structure and function in the process. The document gives a brief over view of Diabetes but not the Biochemistry of it.
-Carlos
references
Pictures
http://thumbs.dreamstime.com/thumblarge_597/1302039175g2x4ig.jpg
Reflection 5. Amino Acids and Proteins: Come and get it..
So let’s begin the quest in Amino Acids and Proteins.…..
What are Amino acids and Proteins?
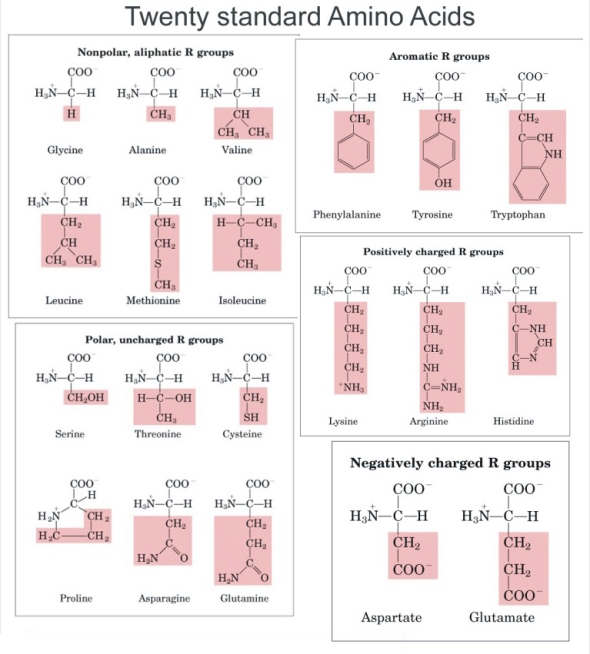
Amino Acids and Proteins were one of the 3 bio-molecules we studied in class. Amino acids are defined as a simple organic compound containing both a carboxyl (COOH) and an amino (NH2) group. Proteins are defined as any of a class of nitrogenous organic compounds that consist of large molecules composed of one or more long chains of amino acids and are an essential part of all living organisms. Amino acids have the basic structure seen in the diagram where R can be given by different groups (highlighted in red). The central carbon is referred to as the Alpha carbon.
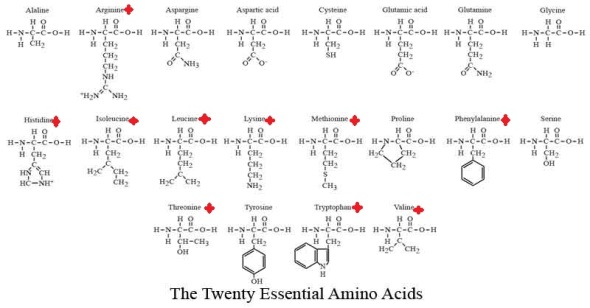
There are 20 essential Amino acids for survival which can be classed as either Essential or Non-Essential Amino Acids.
An Essential amino acid is one that is required by animals that cannot be synthesize by them and must be supplied in the diet. (marked)
Non essential amino acids are those that are synthesized by the body. (unmarked)
Furthermore Essential Amino Acids can be described as either Complete Proteins or Incomplete Proteins. Complete proteins contain all 10 essential amino acids and are derived from animal sources while Incomplete proteins lack one or more of the essential amino acids and most a vegetable based.
How are Amino acids and Proteins classed by structure?
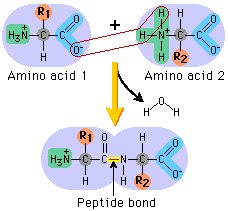
Amino acids bond together to from Proteins. These bonds are called peptide bonds. As a result of bonding Amino acids can be arranged into the following long chain structures;
Peptide– short polymer of amino acids
Di-peptide- contains 2 amino acids joined by a peptide bond.
Tri-peptide– a molecule with 3 amino acids joined by peptide bonds.
Polypeptide– a macro-molecule containing many amino acids.
Protein– a biological molecule of molecular weight 5000g/mol that is made up of polypeptide chains.
How can we identify Amino Acids from Proteins?
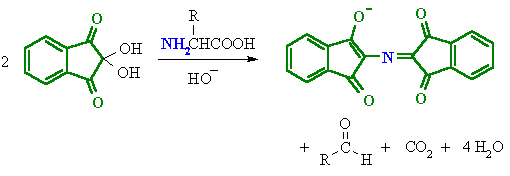
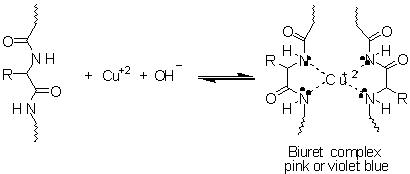
We can use to chemical tests to determine which compounds are amino acids or proteins. These are the Ninhydrin Reaction and the Biuret Test.
Ninhydrin reacts with amino acids to from a Purple colour imino derivative. This derivative is a positive test for amino acids which are commonly colourless.
The Biuret test involves using biuret reagent which is light blue in colour. It contain Copper(II) ion in an alkaline solution. Biuret turns purple when mixed with a solution contain proteins. Biuret reagent interacts with peptide bonds of proteins. The purple colour formed is a positive test for proteins.
How are Proteins classed in terms of structure?
Proteins are classed by the arrangement and the number of polypeptide chains it contains and the level of folding that occur. Protein folding occurs due to Hydrophobic Interactions, Ionic Bonding, Hydrogen bonding and Disulphide bonding. Protein structure can be either Primary, Secondary, Tertiary or Quaternary.
Primary structure: the linear arrangment of amino acids in a protein and the location of covalent linkages such as disulfide bonds between amino acids. These Disulphide bonds are not denatured.
Secondary structure: areas of folding or coiling within a protein; examples include alpha helices and Beta pleated sheets, which are stabilized by hydrogen bonding.
Tertiary structure: the final three-dimensional structure of a protein, which results from a large number of non-covalent interactions between amino acids.
Quaternary structure: non-covalent interactions that bind multiple polypeptides into a single, larger protein. Hemoglobin has quaternary structure due to association of two alpha globin and two beta globin polyproteins.

Protein Structure
Folding can be denatured in several ways.
Heat and Ultra Violet Radiation-Hydrogen bonds are broken by increased translational and vibration energy
Strong Acids/Bases– salt formation, disruption of hydrogen bonds
Urea– competion for hydrogen bonds
Agitation-shared hydrogen bonds
Some Organic Solvents– change in dielectric constant and hydration of ionic groups.
Proteins are essential to body function and are useful in life processes. An example of an important protein structure is an Enzyme.
I do hope this helps with your studies!
-Carlos
References
Websites
-
“Definition of terms .” USA rice. http://www.usarice.com/index.php?option=com_content&view=article&id=629&Itemid=258 (accessed April 13, 2013).
-
“Essential Amino acid.” wordnet web. wordnetweb.princeton.edu/perl/webwn?s=essential%20amino%20acid (accessed April 3, 2013).
-
“The Structure of Proteins.” arbl.cvmbs.colostate.edu. http://www.vivo.colostate.edu/hbooks/genetics/biotech/basics/prostruct.html (accessed April 13, 2013).
Pictures
http://strength-health-alliance.com/wp-content/uploads/2013/02/aminoacids.jpg
http://www.thegeekdoc.com/why-we-believe/20AminoAcids.jpg
http://www.phschool.com/science/biology_place/biocoach/images/translation/peptbond.gif
http://www.chem.ucalgary.ca/courses/351/Carey5th/Ch27/ninhydrin01.gif
http://www.uwlax.edu/faculty/koster/images/imageD2N.JPG
http://www.vivo.colostate.edu/hbooks/genetics/biotech/basics/prostruct.html